Clinical Trials
A History of GP2 Immunotherapy in the Completed Phase IIb Clinical Trial
Encouraging Results
In a prospective, randomized, single-blinded, placebo-controlled, multi-center (16 sites led by MD Anderson Cancer Center) Phase IIb clinical trial of HLA-A*02 breast cancer patients, 46 HER2/neu 3+ over-expressor patients were treated with GLSI-100 and 50 placebo patients were treated with GM-CSF alone. After 5 years of follow-up, there was an 80% or greater reduction in cancer recurrences in the HER2/neu 3+ patients who were treated with GLSI-100 and, followed, as they remained disease free over the first 6 months, which we believe is the time required to reach peak immunity and thus maximum efficacy and protection.
- 80% or greater reduction in metastatic breast cancer recurrence rate over 5 years of follow-up compared to 20-50% reduction in recurrence rate by other approved products
- Peak immunity at 6 months
- No reported serious adverse events attributable to treatment
- Well-tolerated safety profile
Full immunization was received in the Primary Immunization Series (PIS), which included the first 6 GP2 + GM-CSF intradermal injections over the first 6 months. The PIS elicited a potent immune response as measured by local skin tests and immunological assays. Further, booster injections given every 6 months prolonged the immune response, thereby providing longer term protection. In the Phase IIb and three Phase I clinical trials, where 146 patients were treated, the GP2 immunotherapy was well tolerated, and there were no reported serious adverse events related to the GP2 immunotherapy.
Phase IIb Clinical Trial Study Report
The Company is preparing a comprehensive study report of the Phase IIb trial for the FDA prior to the filing of a BLA. This report will include the patients with breast cancer recurrences, the last known date of patients who did not recur (censoring data), the adverse events, immune responses, and other final study report analyses. This report will serve to complement the Phase III data and to provide a drug product dossier that can also be submitted to regulatory agencies in other countries for marketing approval. The use of GM-CSF as an adjuvant in GLSI-100 may also be included in the dossier as GM-CSF is only commercially available in the US at this time.
The Company has experienced significant interest from investors, strategics, analysts, and regulators in the 5 year follow-up data we published and the 3 and 4 year follow-up data independently published by the clinical investigators. The differences between these publications can be best explained by the increased maturity of the data as each year progressed. In all 3 publications, no recurrences or a 100% reduction in recurrence rate, were reported in the sub-population that the Flamingo-01 design has been based on and any differences between the number of patients in the treated or placebo groups has been shown to be immaterial.
The Company did not have responsibility for the conduct of the trial or for the data from the Phase IIb trial. After the trial had already started, the Company received the rights to the Phase IIb trial data pursuant to a license agreement with the Henry Jackson Foundation (HJF) that entitled the Company to all of the GP2 data from the Phase IIb trial and all prior trials, but did not provide the Company with the ability to participate in the Phase IIb trial as a regulatory clinical sponsor. The lead clinicians and HJF were responsible for project and site management, medical monitoring, data monitoring of case report forms (CRFs), correspondence with the FDA, and creation, data entry and management of the database. The Company was provided study updates but was not provided an opportunity to participate in any of the above activities or to review the publications of the 3 and 4 year follow-up data by the lead clinicians. Thus, the comprehensive study report will rely on cooperation from HJF and the clinical sites who are responsible for providing the final data accurately to the Company.
The Company is currently comparing the final CRFs and database provided by HJF and has noted the following inconsistencies as the comprehensive study report is being prepared. The lead clinicians reported in an annual report to the FDA and in their publication of 4 year follow-up data a 6th recurrence in the HER2 positive control arm of the study. The Company conservatively chose not to report this 6th recurrence since it was not reported in the data provided by HJF, even though adding this recurrence to the control arm would significantly lower the p-value and improve the evidence of efficacy of GLSI-100. As a result of detailed due diligence, the Company became aware in Q4 of 2023 of a potential recurrence in the HER2 positive treated arm. This patient was not reported as a recurrence in the database, on a CRF that should be used for a recurrence, in reports from the lead clinicians to the FDA, or in the 3 or 4 year follow-up data published by the lead clinicians. Some CRFs report a recurrence, but the critical CRF that confirms a recurrence was not completed or entered into the database provided by HJF. The Company has since initiated an effort to confirm with HJF and the clinicians who treated this patient the status of this patient, and if the final CRFs and database should be modified. It appears that this patient, who had completed treatment with GLSI-100, experienced a local recurrence that responded well to additional treatment and survived without additional evidence of disease or distant metastasis for the duration of study follow-up. Any discrepancies noted to date in the review of the censoring date recorded in the database do not materially change the study results and the median duration of follow-up remains 5 years.
While a recurrence in the control arm would decrease the p-value and still result in a 100% reduction in the recurrence rate, a recurrence in the treated arm would increase the p-value and would result in an 80% reduction in the recurrence rate. In either case, the Company believes that the reduction in recurrence rate is clinically meaningful and substantial compared to the approximately 20-50% reduction in recurrences of all other approved breast cancer drugs for this patient population. These findings have not materially affected the power of the Phase III study as the assumptions for that design were selected conservatively.
Phase IIb Clinical Trial 5 Year Breast Cancer Recurrence Rate Final Results — SABCS 2020
Poster PS10-23: GP2 5 Year Top-Line Data (view PDF here)
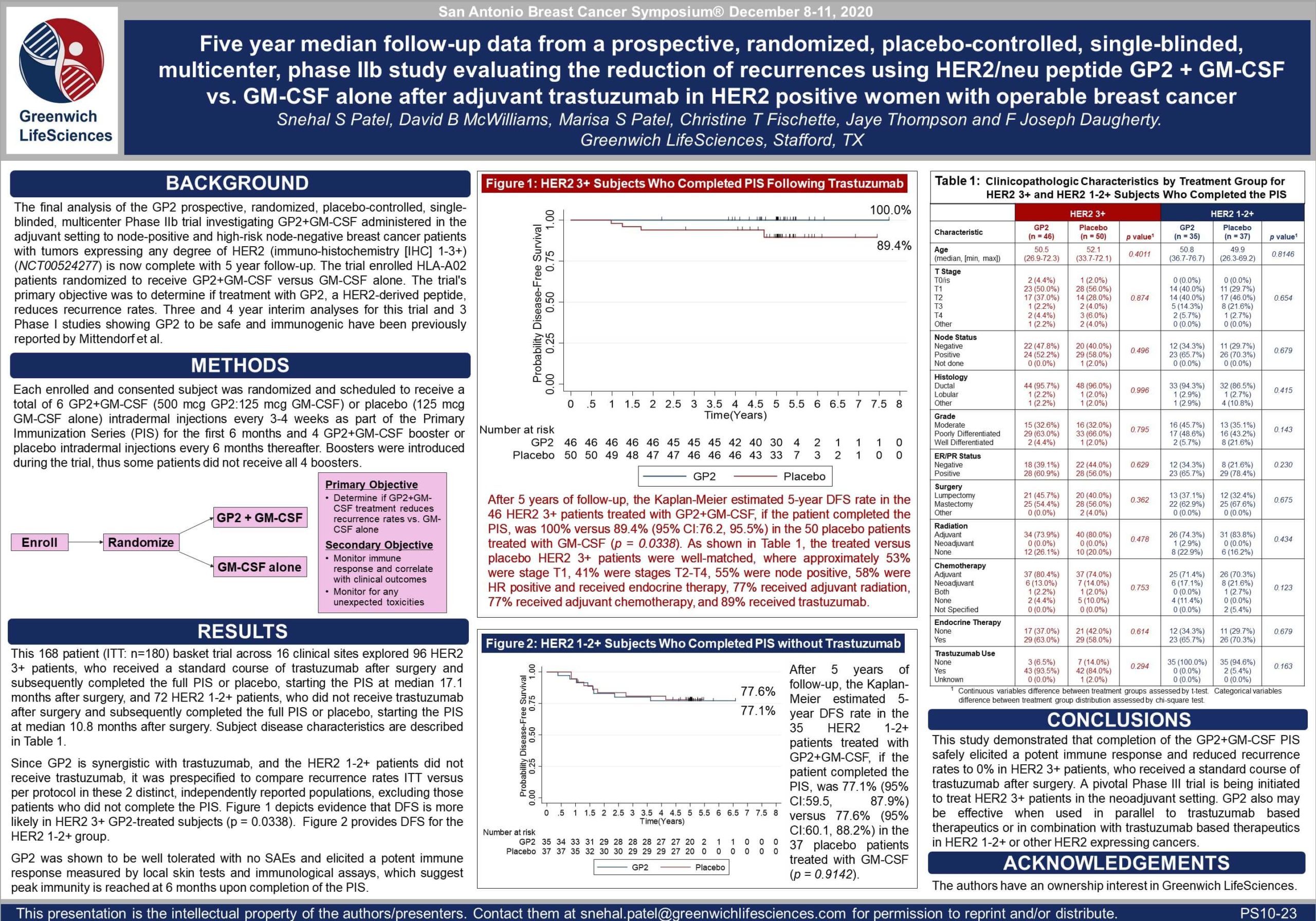
Table 1: The treated versus placebo HER2 3+ patients were well-matched, where approximately 53% were stage T1, 41% were stages T2-T4, 55% were node positive, 58% were hormone receptor positive and received endocrine therapy, 77% received adjuvant radiation, 77% received adjuvant chemotherapy, and 89% received trastuzumab. There were no recurrences in the 10-11 HER2 3+ patients who did not receive trastuzumab.
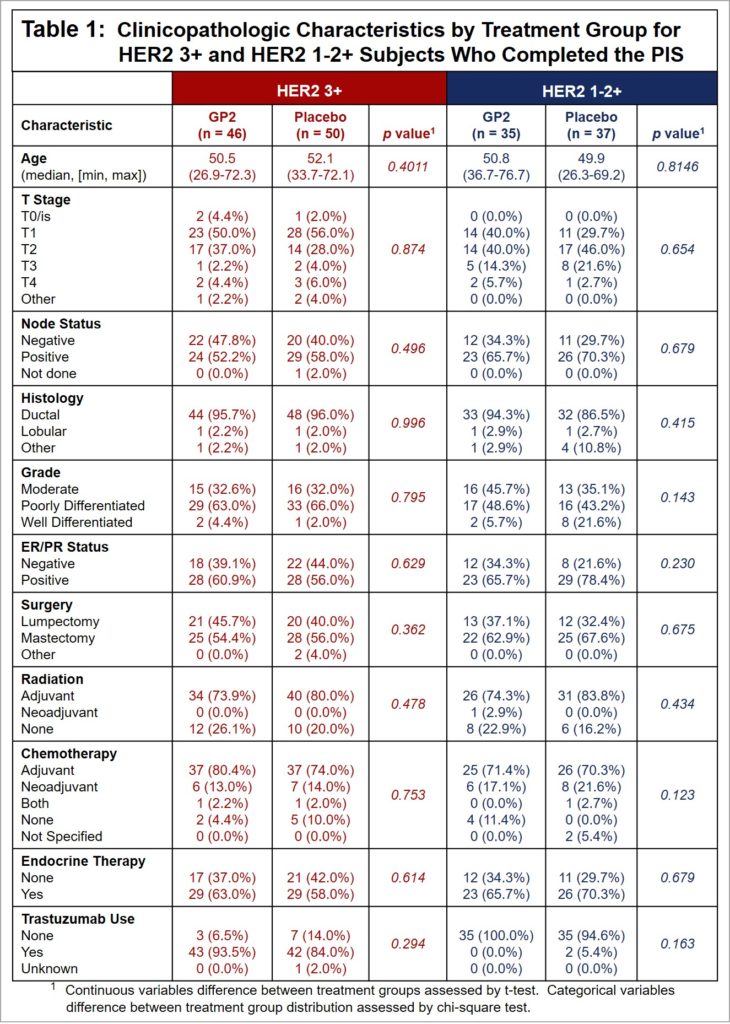
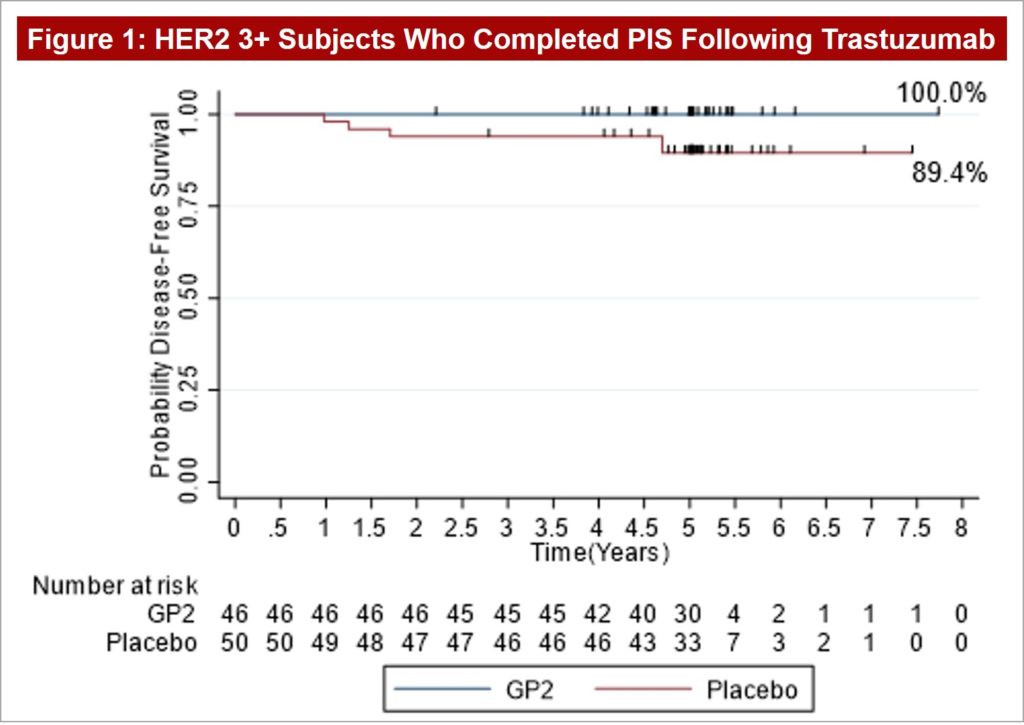
Figure 1: After 5 years of follow-up, the Kaplan-Meier estimated 5-year DFS rate in the 46 HER2 3+ patients treated with GP2+GM-CSF, if the patient completed the PIS, was 100% versus 89.4% (95% CI:76.2, 95.5%) in the 50 placebo patients treated with GM-CSF (p = 0.0338).
Press Release on December 9, 2020: “Greenwich LifeSciences Announces Poster Presentation of Five Year Data for GP2 Phase IIb Clinical Trial, Showing 0% Recurrence of Breast Cancer”
Phase IIb Clinical Trial 5 Year Immune Response Final Results — AACR 2021
Poster CT183: GP2 5 Year Immune Response Data (view PDF here)
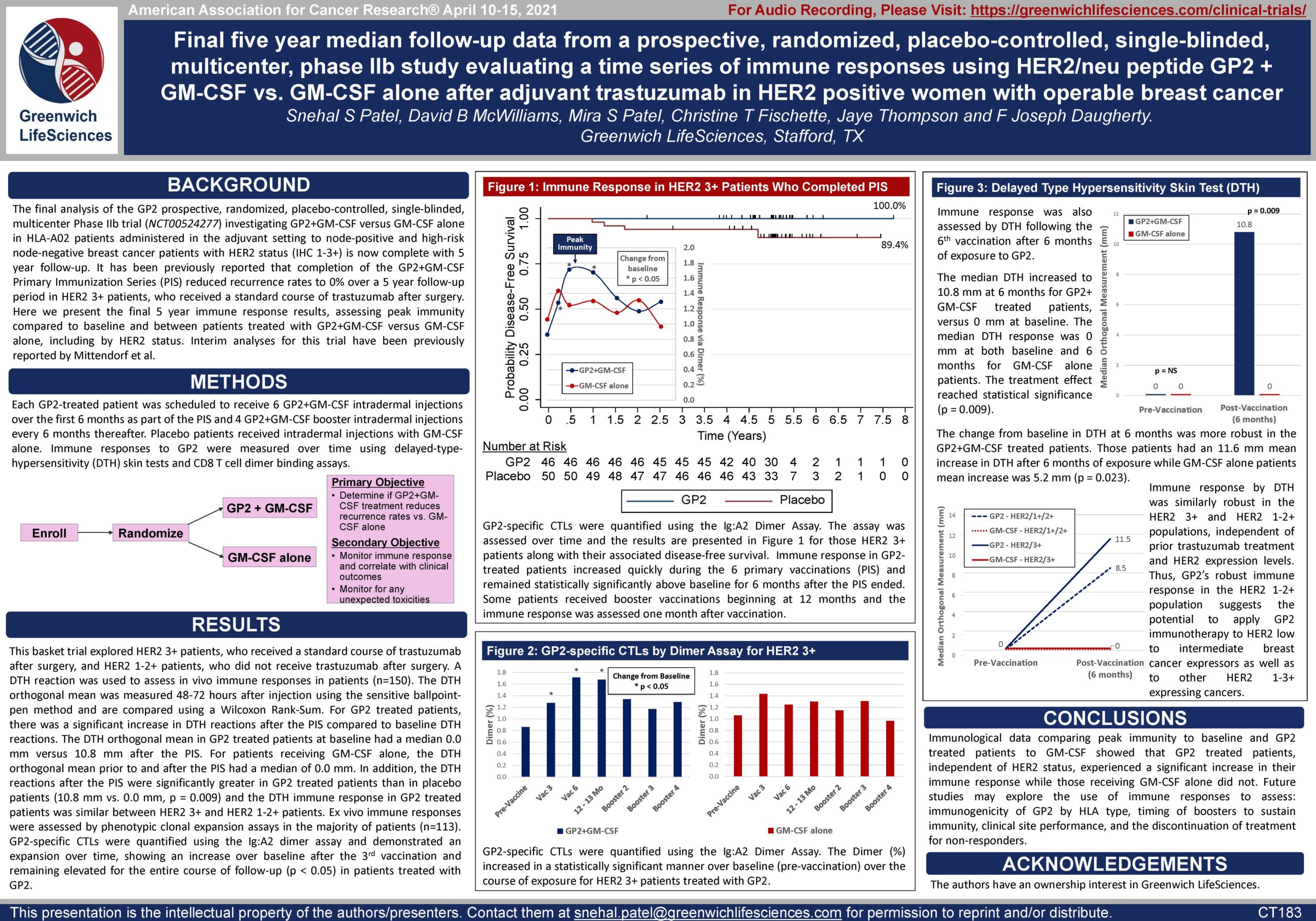
Figures 1-3: Immune Response Measured by Dimer Binding Assay and DTH Skin Test
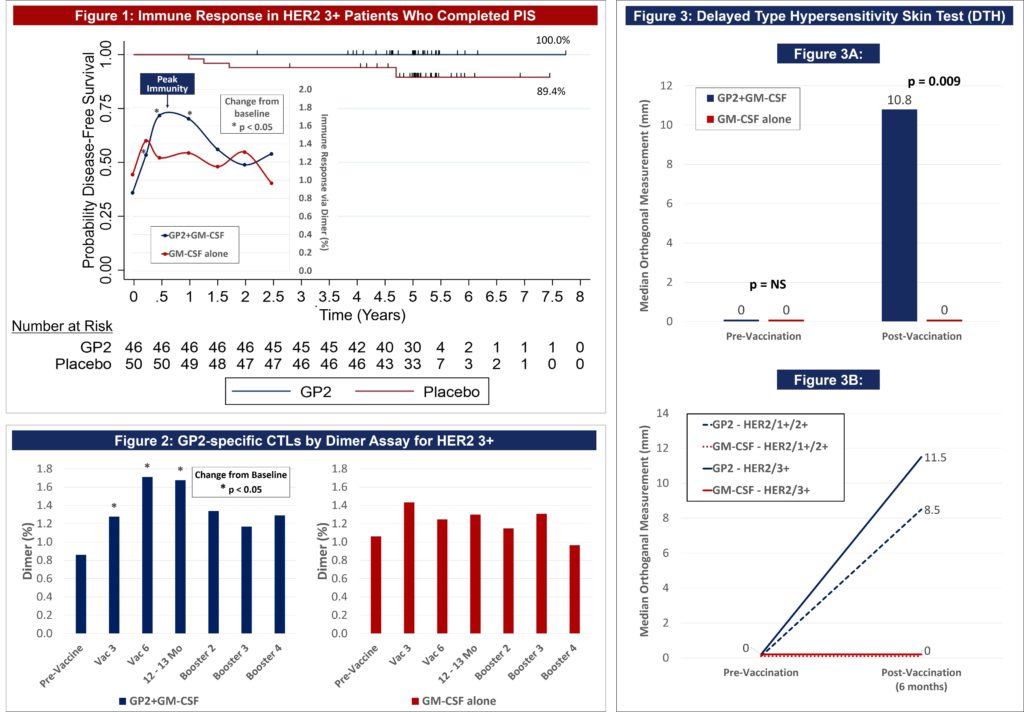
Figure 1: The data shows that GP2 immunity peaked at 6 months in HER2 3+ patients after they completed their first 6 immunizations, as measured by the Dimer Binding Assay. The data also shows that for the 2.5 years that the immune response was measured, the immunity was sustained and remained above baseline, resulting in 100% disease free survival (0% recurrence rate) over 5 years. In the placebo arm, the immune response was not as robust, resulting in 89% disease free survival (11% recurrence rate). Immune response in GP2-treated patients increased quickly during the Primary Immunization Series and remained statistically significantly above baseline for 6 months after the completion of the Primary Immunization Series. Some patients received boosters beginning at 12 months and the immune response was assessed one month after the receiving the booster.
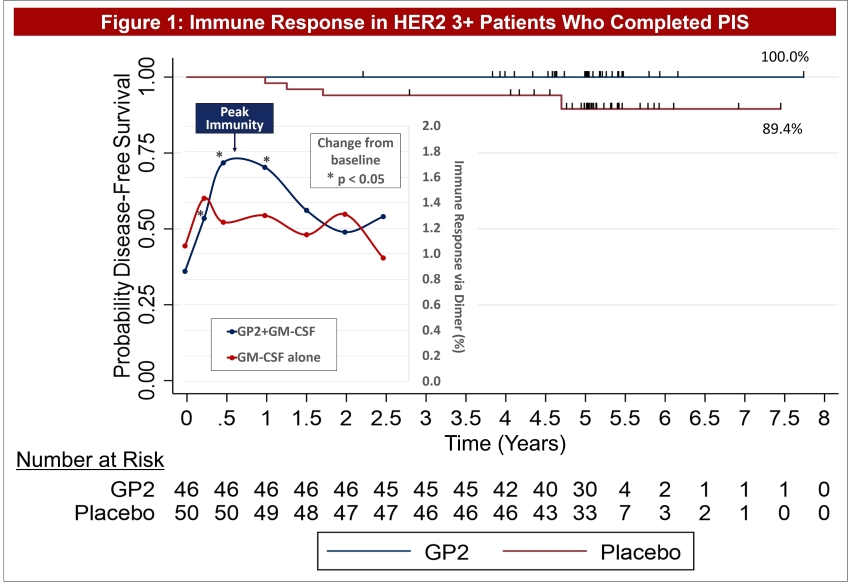
Figure 2: The same Dimer Binding Assay data for HER2 3+ patients is shown as in Figure 1, where the GP2 treated patients showed statistically significant dimer readings versus baseline (pre-vaccination) at 3, 6, and 12-13 months.
Dimer Binding Assay: The Dimer Binding Assay detects the percentage of GP2 specific killer T cells that can kill recurring cancer cells. Ex vivo immune response was assessed over 2.5 years with blood draws at baseline, then after the 3rd and 6th immunizations in the Primary Immunization Series, and then after each booster. Immune responses were assessed by phenotypic clonal expansion assays in the majority of patients (n=113). GP2-specific CTLs were quantified in patients treated with GP2 using the Ig:A2 Dimer Assay and demonstrated an expansion over time, showing an increase over baseline after the 3rd immunization and remaining elevated for the entire course of follow-up.
DTH Skin Test: The DTH skin test measures the diameter of the skin immune response to GP2 in millimeters, 48-72 hours after intradermal injection of GP2 without GM-CSF. A DTH reaction was used to assess in vivo immune responses in patients (n=150). The DTH orthogonal mean of the skin wheal was measured 48-72 hours after injection using the sensitive ballpoint-pen method and is compared using a Wilcoxon Rank-Sum. For GP2 treated patients, there was a significant increase in DTH reactions after the PIS compared to baseline DTH reactions.
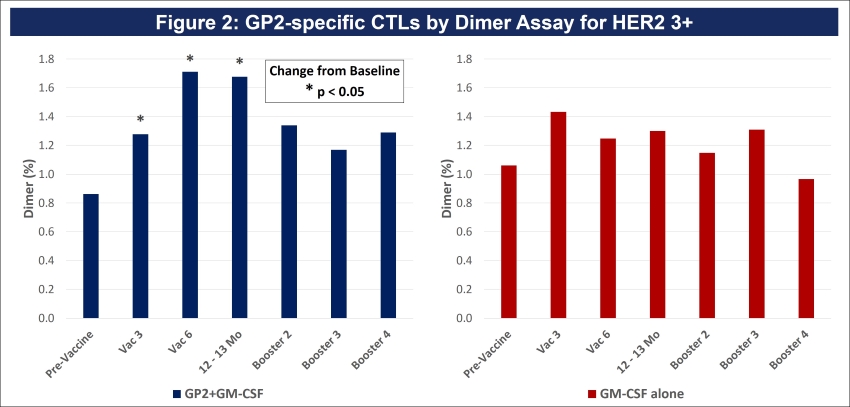
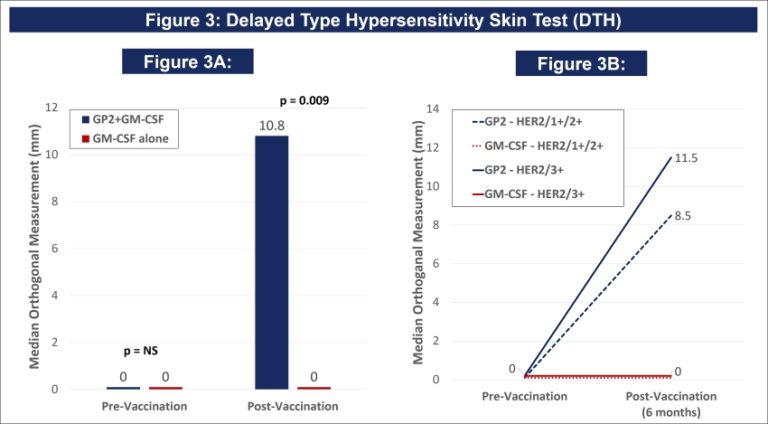
Figure 3A: After completion of the 6th immunization after 6 months, GP2 treated patients showed a robust immune response using the DTH skin test, while the placebo did not (p = 0.009). Within GP2 treated patients, the change from baseline after 6 months was a median of 4.8 mm (mean of 11.6 mm), which was a statistically significant increase over baseline (p < 0.0001). The change from baseline in DTH at 6 months was more robust in the GP2 treated patients. Those patients had an 11.6 mm mean increase in DTH after 6 months of exposure while patients treated with GM-CSF alone had a 5.2 mm mean increase (p = 0.023). This DTH data supports the Dimer Binding Assay data that shows a peak immune response after 6 months.
Figure 3B: The DTH immune response for GP2 treated patients was similarly robust in HER2 3+ patients and HER2 1-2+ patients, independent of prior trastuzumab treatment and HER2 expression levels. Thus, GP2’s robust immune response in the HER2 1-2+ population suggests the potential to apply GP2 immunotherapy to HER2 low to intermediate expressing breast cancers, as well as to other HER2 1-3+ expressing cancers.
Press Release on April 10, 2021: “Greenwich LifeSciences Presents Immune Response Phase IIb Poster […] Showing Peak Immunity after 6 Months of GP2 Treatment, Resulting in 100% Disease Free Survival from Recurring Breast Cancer”
Phase IIb Clinical Trial 5 Year Safety Final Results — ASCO 2021
Poster 542: GP2 5 Year Safety Data (view PDF here)
Figure 1: The incidence of AEs in the HER2 3+ population over time are presented in Figure 1. Injection site reactions account for the majority of reported events and therefore are temporally associated with vaccinations. Incidence of AEs for GP2+GM-CSF patients and GM-CSF only patients are quite similar for the duration of follow-up.
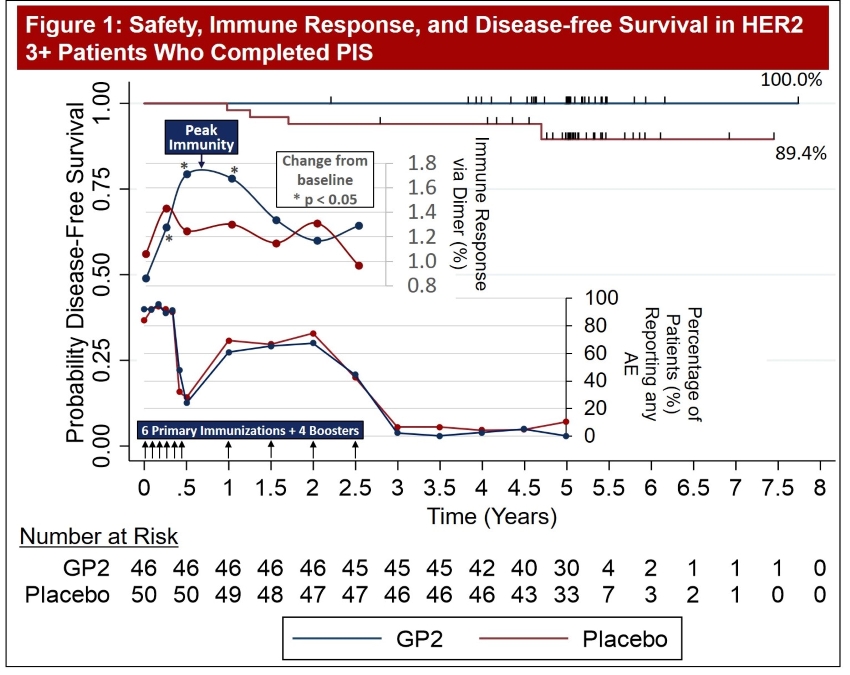
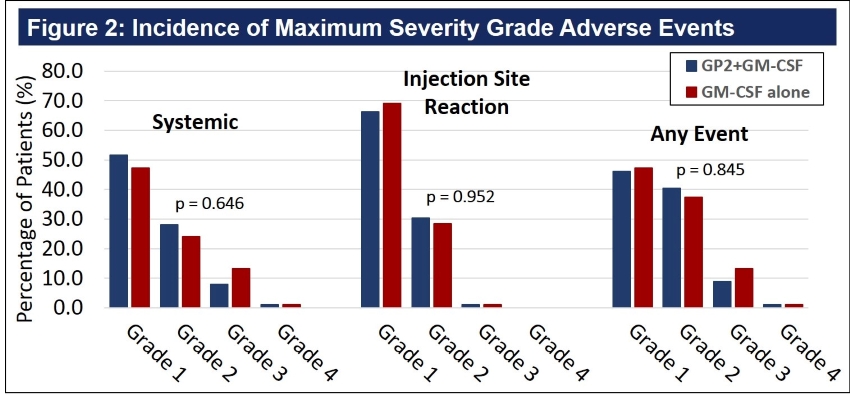
Figure 2: The maximal severity grade for any AE, systemic and injection site reaction, for each patient was identified. There was no difference between the two treatment arms, as presented in Figure 2. The majority of events were of grade 1, mild severity. Two patients reported grade 4 AEs deemed unrelated to study medication. One GP2+GM-CSF patient experienced grade 4 hypoglycemia and recovered. A GM-CSF only patient was diagnosed with renal cell carcinoma, a second primary diagnosis, which was classified as grade 4.
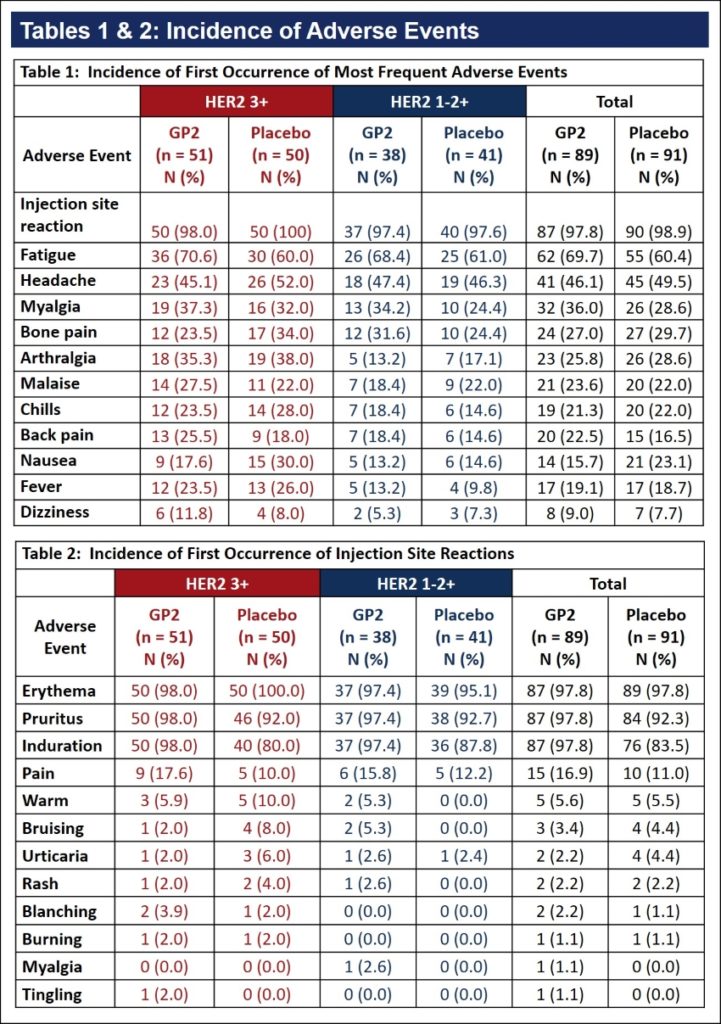
Tables 1 & 2:
The first occurrence of frequently reported AEs is tabulated in Table 1. The most common AE was injection site reaction. Almost every patient, in both the GP2+GM-CSF and GM-CSF only arms, reported injection site reactions.
The most frequent injection site reactions were erythema, pruritus and induration, as presented in Table 2. The incidence in AEs was similar across HER2 3+ and HER2 1-2+ patients.
No serious adverse events considered related to study medication were reported.
Press Release on June 7, 2021: “Greenwich LifeSciences Publishes Additional Positive Safety Data from GP2 Phase IIb Trial at ASCO 2021, Confirming that GP2 Treatment to Prevent Metastatic Breast Cancer Recurrence is Well Tolerated”
Phase IIb Clinical Trial Baseline Immune Response Data — SABCS 2021
Poster P2-13-29: GP2 Baseline Immune Response Data (view PDF here)

Figure 1: Some patients reported significant DTH responses at baseline, prior to exposure to GP2. It was found that 22.8% of patients reacted to GP2 at baseline with induration of 5 mm of greater in the baseline DTH test. A positive response is defined as a site measurement of 5 mm of larger.
It was noted that some patients reported significant DTH responses at baseline, prior to exposure to GP2. It was found that 22.8% of patients reacted to GP2 at baseline with induration of 5 mm or greater in the baseline DTH test. A positive response is defined as a site measurement of 5 mm or larger.
A Kaplan-Meier analysis in the subgroup of patients who experienced a recurrence of breast cancer over the course of the study investigates time to recurrence by baseline DTH response (positive = Baseline Response / negative = None). Although the survival curves become quite similar after 1.5 years, which makes the two curves statistically the same, p = 0.7140, the early effect of baseline response to GP2 assessed by DTH creates an obvious effect on early recurrence. The median time to recurrence of those with a baseline DTH response is 99 days (0.27 years) while those with no baseline DTH took 438 days (1.2 years) to recur by life table analysis techniques. These summary statistics by standard methods are 0.6 years for those with a baseline response and 1.2 years for those with none.
Note, these analyses are conducted independent of the treatment group assigned as the interest was how baseline DTH results might predict early recurrence.
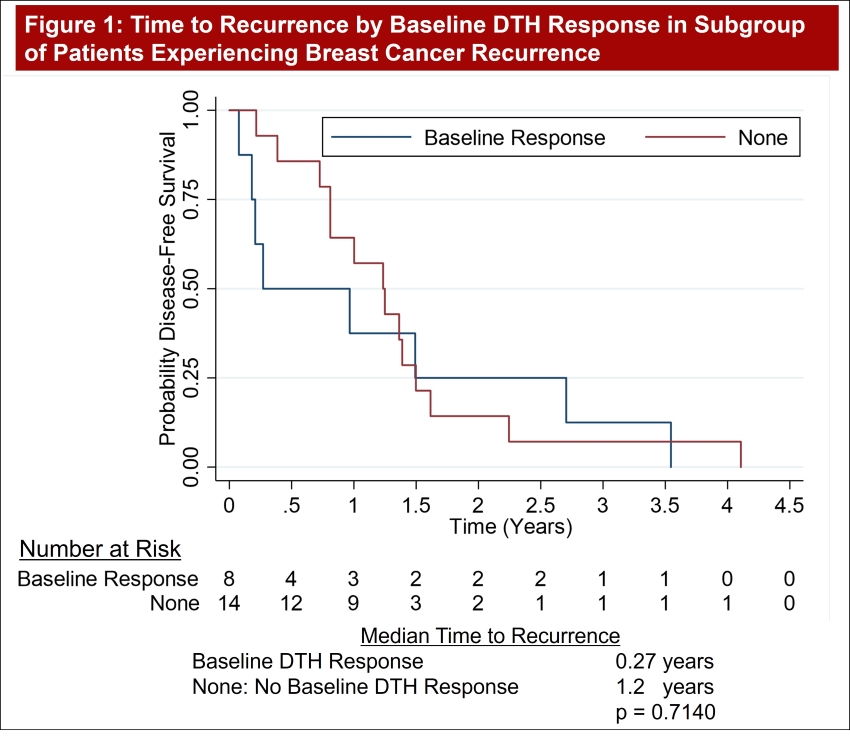
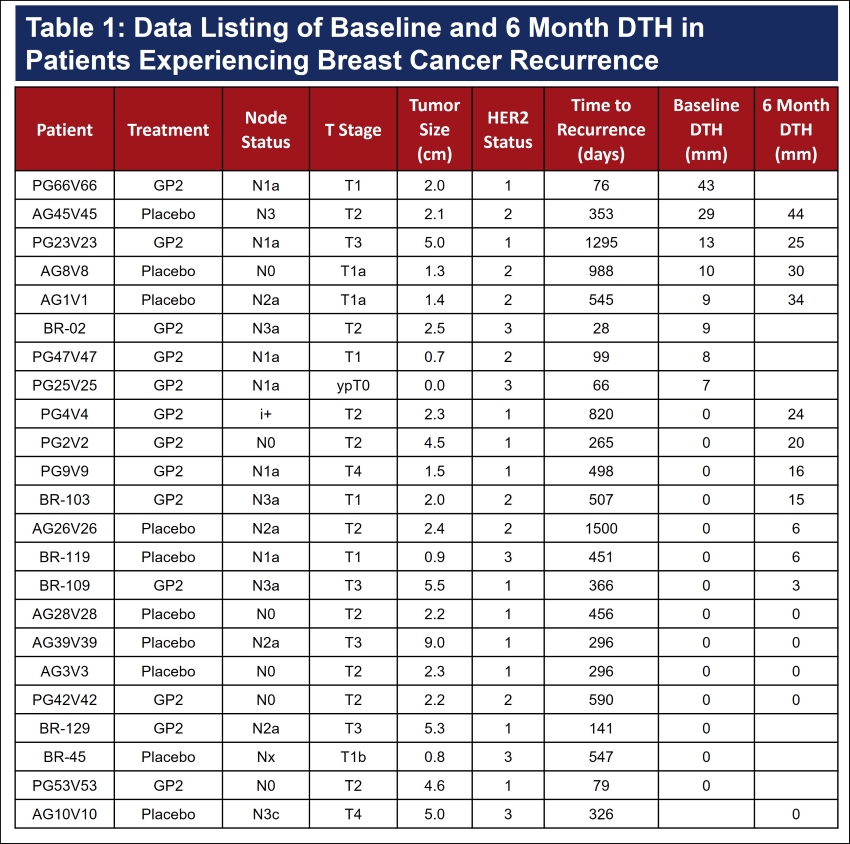
Table 1: Raw data for the subjects who recurred over the course of follow-up and their baseline and 6 month DTH assessments.
Press Release on December 9, 2021: “Greenwich LifeSciences Announces Presentation of 5 Year Data for GP2 Phase IIb Clinical Trial, Revealing Potential For New T Cell Platform Technology”
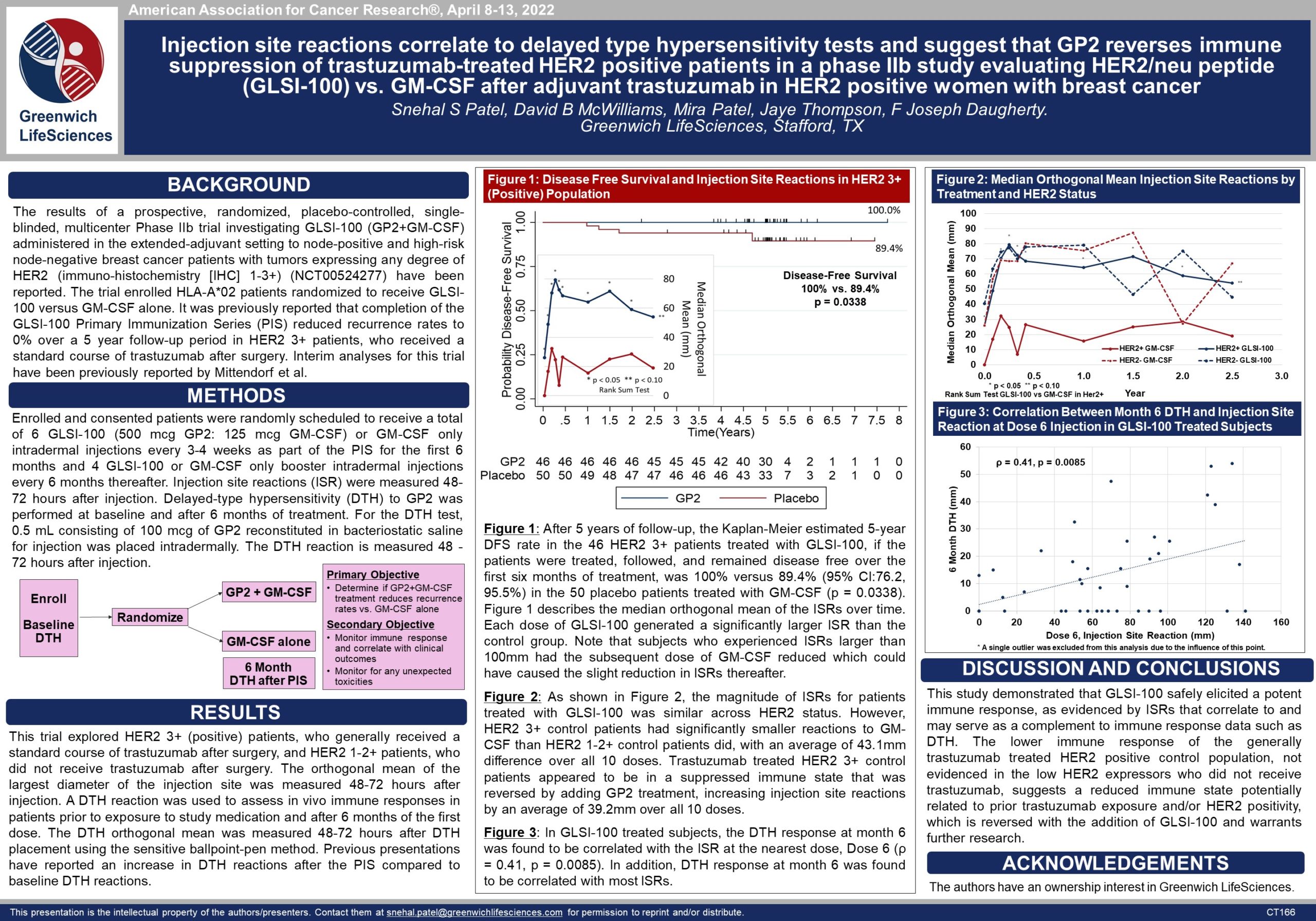
Phase IIb Clinical Trial GP2 Reverses Immune Suppression — AACR 2022
Poster CT166: GP2 Reverses Immune Suppression (view PDF here)
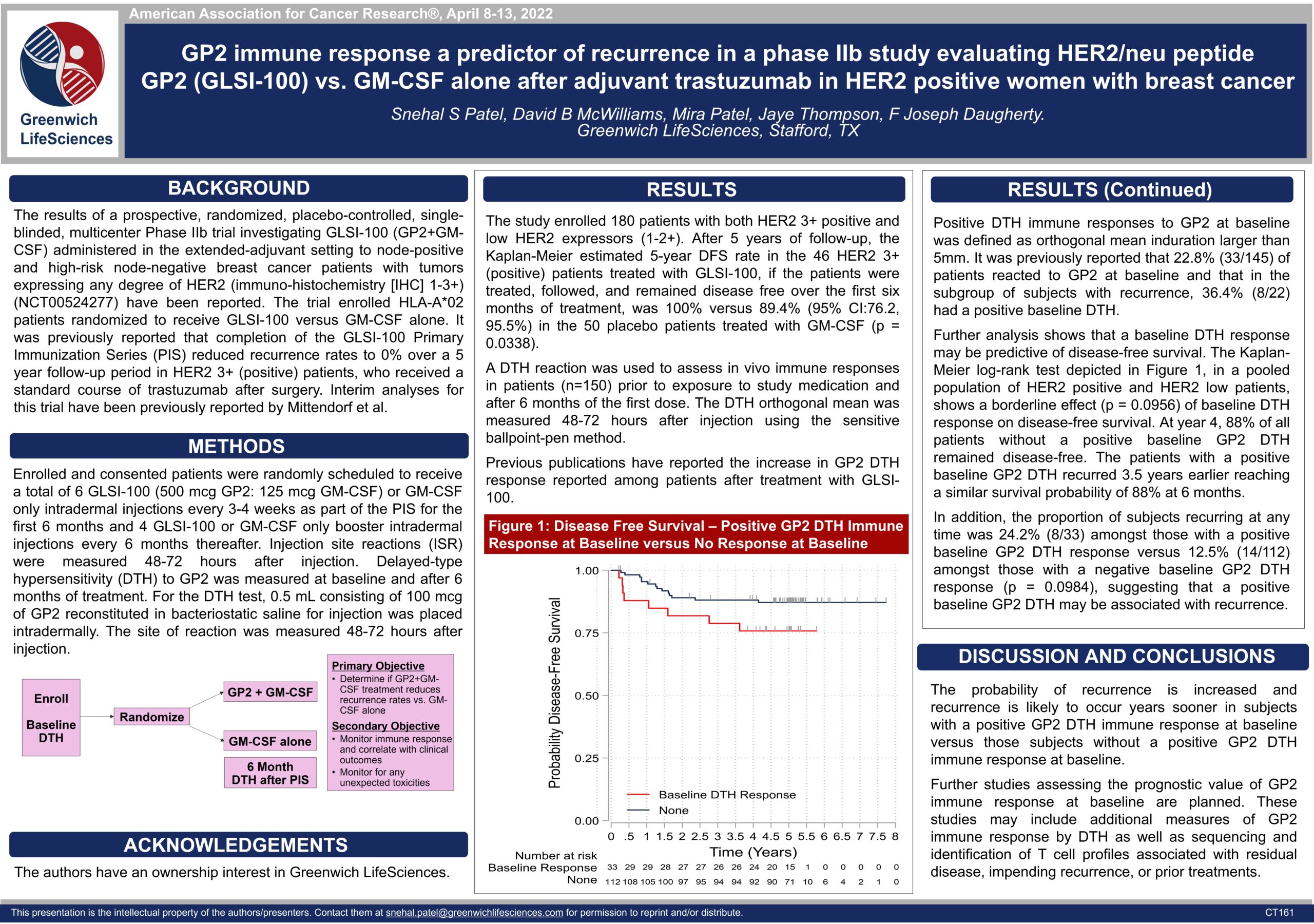
Phase IIb Clinical Trial GP2 Baseline Immune Response Predicts Recurrence — AACR 2022
Poster CT161: GP2 Baseline Immune Response Predicts Recurrence (view PDF here)
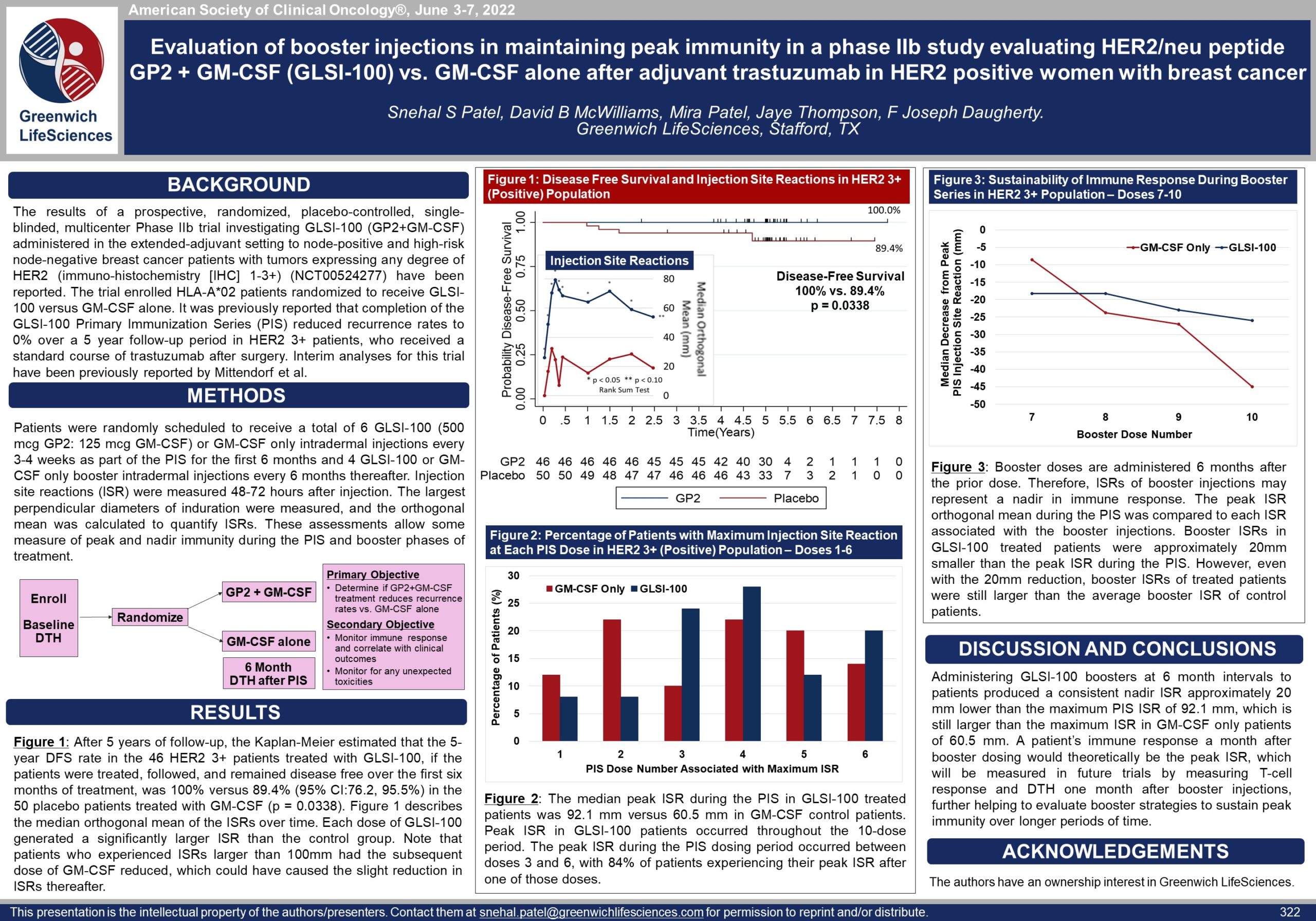
Phase IIb GP2 Clinical Trial Evaluation of Booster Injections in Maintaining Immunity — ASCO 2022
Poster 322: Evaluation of GP2 Booster Injections in Maintaining Immunity (view PDF here)