Welcome to
Greenwich LifeSciences
Currently in a Phase III Clinical Trial at 150 Sites in U.S. and Europe
to Prevent Recurring Breast Cancer
Greenwich LifeSciences (Nasdaq: GLSI) is a clinical-stage biopharmaceutical company, pioneering an immunotherapy vaccine called GLSI-100, which is designed to activate the immune system in preventing breast cancer recurrences in HER2-positive patients.



GLSI-100 is a first-of-its-kind therapy that combines GP2, a HER2/neu-derived peptide, with GM-CSF.
With 1 in 8 U.S. women developing invasive breast cancer over her lifetime, 9.5 million breast cancer survivors per year, and an estimated 700,000 new breast cancer cases per year, in the U.S. and Europe, alone, GLSI-100 addresses a critical unmet need in cancer care.
Safety and Efficacy
Shown in 146 Treated Breast Cancer Patients
from 3 Completed Phase I Clinical Trials and
1 Completed Phase IIb Clinical Trial
In the completed Phase IIb Trial, led by MD Anderson Cancer Center at 16 sites, the initial data, summarized below, was announced at the SABCS conference on December 9, 2020, leading to a $158 intraday share price and 3000% gain from its prior day close.
- 80% or greater reduction in metastatic breast cancer recurrence rate over 5 years of follow-up compared to 20-50% reduction in recurrence rate by other approved products
- Peak immune response at 6 months
- No reported serious adverse events attributable to treatment
- Well-tolerated safety profile
Greenwich LifeSciences is currently advancing GLSI-100 through its global Phase III clinical trial, called Flamingo-01, in which up to 750 patients are planned to be treated. In alignment with top-tier research networks — US (US Oncology, TRIO US), Spain (GEICAM), Italy (GIM), France (Unicancer), Germany (GBG), and Polish network — and prestigious institutions, across 150 clinical sites, Greenwich LifeSciences is striving to build on the promising results of the Phase IIb clinical trial.
GLSI-100 is positioned to be a breakthrough therapy, with the potential to reduce metastatic breast cancer recurrence rates by more than the ~50% achieved by existing HER2 therapies, such as Herceptin and Kadcyla. This exceptional efficacy is paired with a strong safety profile, validated by ongoing Data Safety Monitoring Board recommendations to continue the Phase III trial without modifications.
Flamingo-01 Phase III Clinical Trial
Overview
Flamingo-01 is a prospective, randomized, double-blinded, multi-center study. Its primary objective is to assess the safety and efficacy of GP2 compared to placebo in HLA-A*02 positive and HER2/neu positive breast cancer patients, who had a high risk of disease recurrence and have completed both neoadjuvant and postoperative, adjuvant trastuzumab-based standard of care therapy.
As currently designed, approximately 500 HLA-A*02 patients will be randomized to receive GLSI-100 or placebo in the first two pivotal arms of the trial, with a planned interim analysis. The planned interim analysis is conservatively designed for a 70% reduction in breast cancer recurrence, based on the prior Phase IIb results that showed an 80% or greater reduction in recurrence rate, in comparison to the ~50% recurrence reduction achieved by existing HER2-targeted therapies like Herceptin and Kadcyla. The primary endpoints of the pivotal arms will be blinded, but various other data — such as immune response and safety — may be open-label. In addition, up to 250 non HLA-A*02 patients will be enrolled in the third open-label arm, where all patients will receive GLSI-100 and where all endpoints will be open-label.
For general questions and participation interest, email flamingo-01@greenwichlifesciences.com.
For clinical site contact information and an interactive map of the 150 clinical sites, visit the clinicaltrials.gov’s Flamingo-01 page under its “Contacts and Locations” section.
Steering Committee
The Flamingo-01 Steering Committee is comprised of the following members, who are recognized breast cancer key opinion leaders representing major clinical networks and prominent hospitals in the U.S. and Europe:
- Dr. Mothaffar F. Rimawi – Baylor
- Dr. Francois-Clement Bidard – Unicancer (France)
- Dr. William J. Gradishar – Northwestern
- Dr. Sibylle Loibl – GBG (Germany)
- Dr. Miguel Martin – GEICAM (Spain)
- Dr. Joyce A. O’Shaughnessy – US Oncology
- Dr. Hope S. Rugo – University of California, San Francisco
- Dr. Laura M. Spring – Harvard
- Dr. Cesar A. Santa-Maria – Johns Hopkins
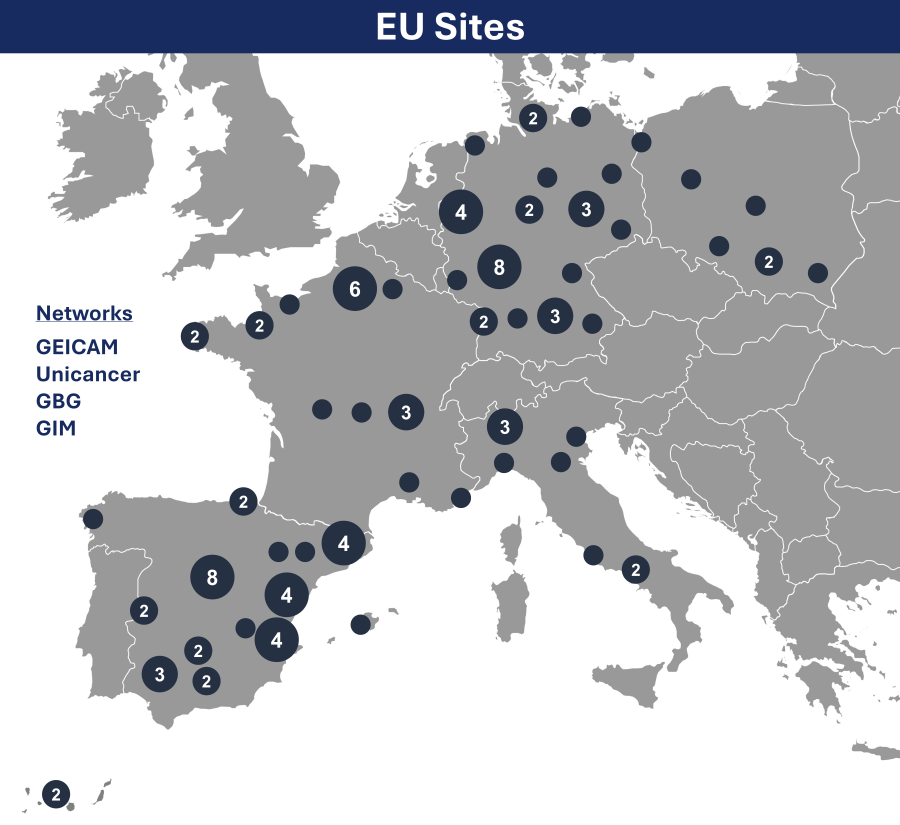
Sites
A map showing the majority of participating Flamingo-01 clinical sites is summarized, below.

Investment Highlights
Market-leading breast cancer recurrence reduction rate from the Phase IIb Trial Results, with a promising efficacy and safety profile.
Ongoing global Phase III clinical trial expansion with planned Interim Analysis.
Robust clinical oversight and rigorous clinical trial execution, led by a distinguished Steering Committee of globally-recognized oncology experts and Management & Board of veteran biopharmaceutical industry experts.
Potential Phase II clinical trial opportunities to expand the market, including but not limited to HER2/neu 1-2+ patients with Herceptin, other HLA types, combinations with CD4/CD8 peptides and checkpoints, and other HER2/neu cancers.
Strong manufacturing readiness with up to ~200,000 doses of commercial GP2 product available prior to filing of BLA.
Two-time “Russell 2000 Index” member with commitment to shareholder interests, evident in its Management & Board ownership over 50% and extended lock-up agreements approaching 5 years since IPO.
