Our Cancer Immunotherapy
A Product Description of GLSI-100
Following breast cancer surgery, a HER2/neu positive patient receives trastuzumab (Genentech/Roche’s Herceptin, a monoclonal antibody treatment that targets the HER2/neu protein) in the first year and then hopes that their breast cancer will not recur, with the odds of recurrence slowly decreasing over the first 5 years. Our immunotherapy, GP2, may be synergistic with Herceptin.
GLSI-100 combines GP2 with GM-CSF. GP2 is a nine amino acid transmembrane peptide of the HER2/neu protein. HER2/neu is a cell surface receptor protein that is expressed in 75% of breast cancer in addition to a variety of other common cancers. Following GLS-100 immunotherapy treatment, CD8+ cytotoxic T lymphocytes (“CTLs”) recognize and destroy HER2/neu-expressing cancer cells. GP2 is administered in combination with GM-CSF, an FDA-approved immunoadjuvant, which stimulates the proliferation of antigen presenting cells.
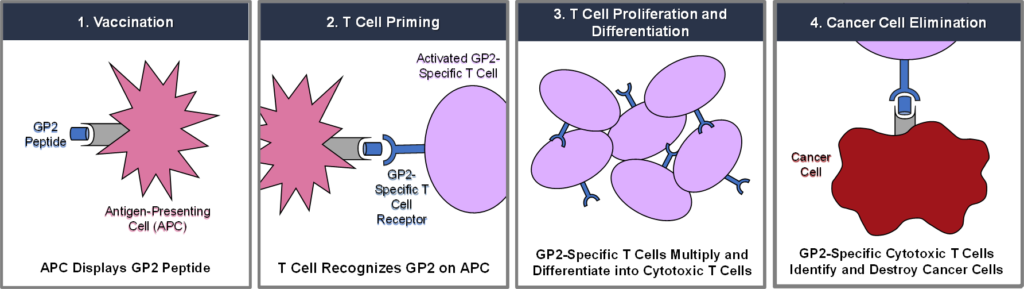
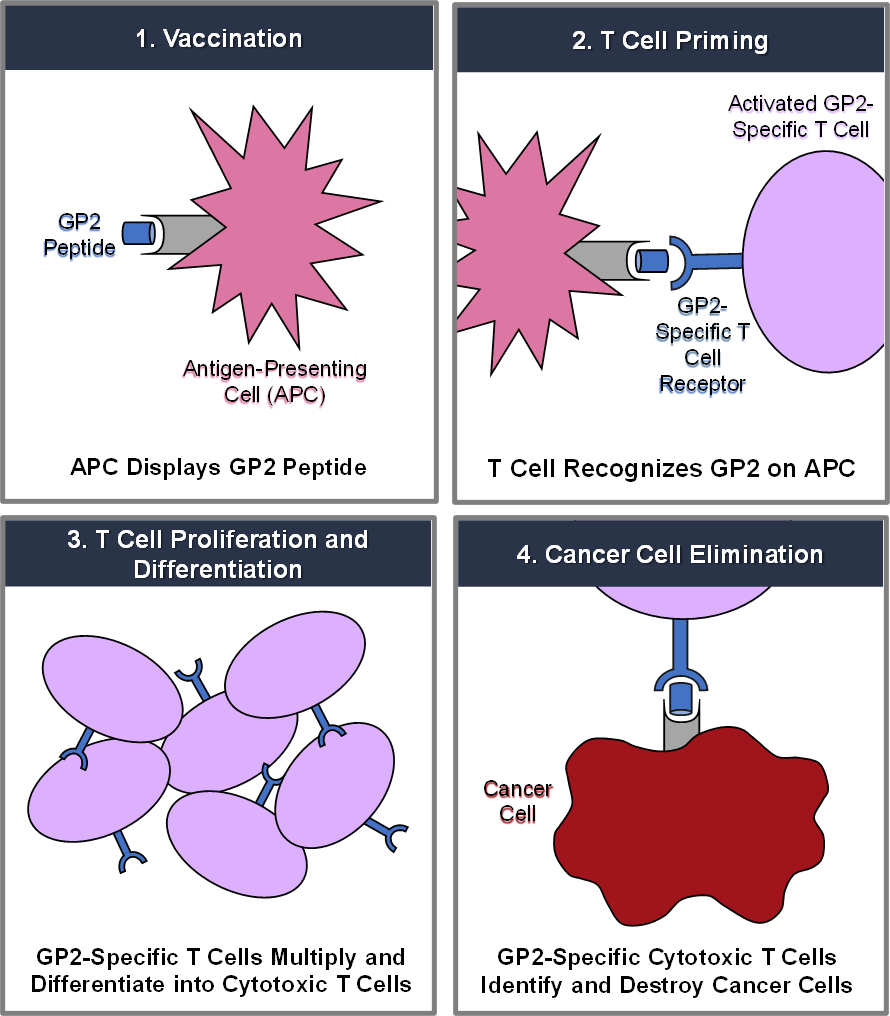
Preclinical studies have shown that T cells sensitized against the GP2 peptide demonstrate significant recognition of HER2/neu-expressing tumors. Both ovarian and breast cancer-specific CTLs recognize GP2, which is widely expressed in HER2/neu-expressing tumors and is capable of inducing tumor-specific CTL populations in vitro.
Four clinical trials have concluded, and a Phase III clinical trial, Flamingo-01, has commenced, to explore GLSI-100 as an immunotherapy for preventing the recurrence of breast cancer following surgery.
