Clinical Trials
The Flamingo-01 Phase III Clinical Trial
The Phase III clinical trial, Flamingo-01, has commenced.
Flamingo-01 Steering Committee
The Flamingo-01 Steering Committee is comprised of the following members:
- Dr. Mothaffar F. Rimawi – Professor of Medicine at the Baylor College of Medicine and Executive Medical Director and Co-Leader, Breast Cancer Program of the Dan L Duncan Comprehensive Cancer Center
- Dr. Francois-Clement Bidard – Professor of Medical Oncology, UVSQ/Paris Saclay University, Head of Breast Cancer Group, Institut Curie, Vice-Chair of the French Breast Cancer research group UCBG (Unicancer)
- Dr. William J. Gradishar – Professor of Medicine at the Feinberg School of Medicine at Northwestern University, Chief of Hematology and Oncology in the Department of Medicine, and Betsy Bramsen Professor of Breast Oncology
- Dr. Sibylle Loibl – Professor (apl) Goethe University Frankfurt/M, Clinical Consultant Centre for Haematology and Oncology/Bethanien Frankfurt/M, CEO of GBG Forschungs GmbH & Chair of the German Breast Group (GBG)
- Dr. Miguel Martin – Professor of Medicine, Head, Medical Oncology Service, Gregorio Marañón General University Hospital, Complutense University, Madrid, CEO of GEICAM
- Dr. Joyce A. O’Shaughnessy – Celebrating Women Chair in Breast Cancer, Baylor University Medical Center and Chair, Breast Cancer Program, Texas Oncology, US Oncology, Dallas, Texas
- Dr. Hope S. Rugo – Professor of Medicine and Winterhof Family Professor of Breast Oncology and Director, Breast Oncology and Clinical Trials Education, University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center
- Dr. Laura M. Spring – Assistant Professor, Medicine, Harvard Medical School, Attending Physician, Medical Oncology, Massachusetts General Hospital
- Dr. Cesar A. Santa-Maria – Associate Professor of Oncology, Breast and Gynecological Malignancies Group, Director of Breast Cancer Trials, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center
Flamingo-01 Overview
Flamingo-01 has a planned interim analysis, using a similar treatment regimen as the completed Phase IIb clinical trial.
The primary objective of Flamingo-01 is to to assess the safety and efficacy of GP2 compared to placebo in HLA-A*02 positive and HER2/neu positive breast cancer patients, who had a high risk of disease recurrence (Stage I, Stage II, or Stage III at presentation, with residual disease at surgery; or, Stage III at presentation, with pathologic complete response at surgery) and have completed both neoadjuvant and postoperative, adjuvant trastuzumab-based standard of care therapy.
Trial details are available at clinicaltrials.gov:
A map showing the majority of participating clinical sites can be viewed, below. An interactive version can be viewed on large screens at clinicaltrials.gov, under the “Contacts and Locations” section.

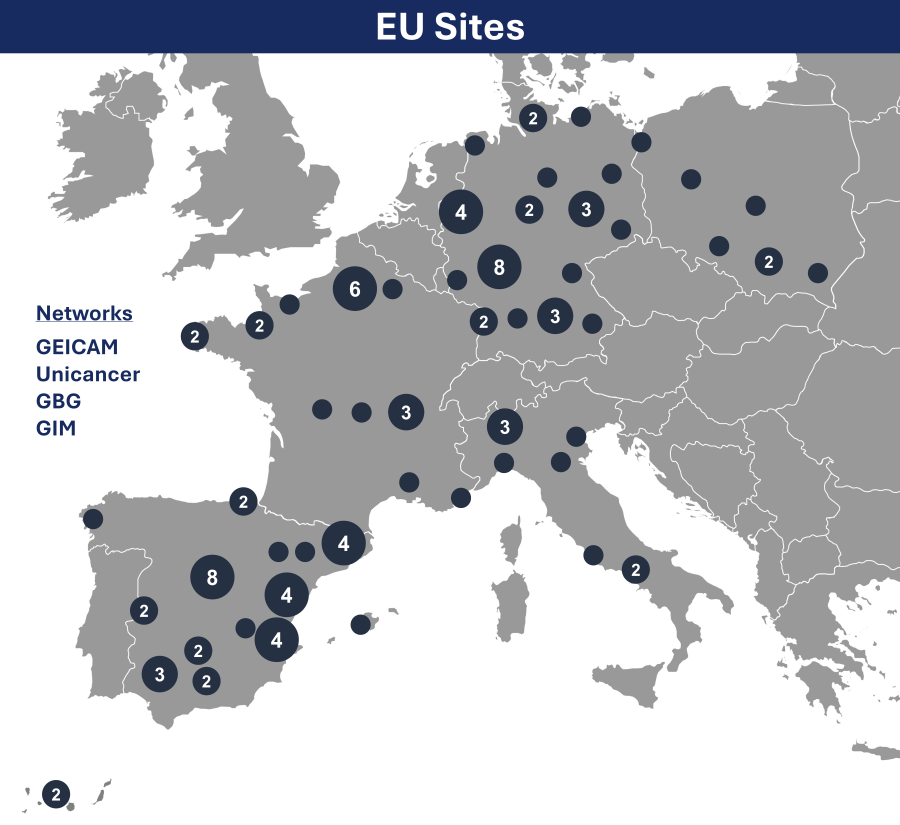
Clinical site contact information can also be viewed at clinicaltrials.gov, under the “Contacts and Locations” section. Related general questions and participation interest can be emailed to:
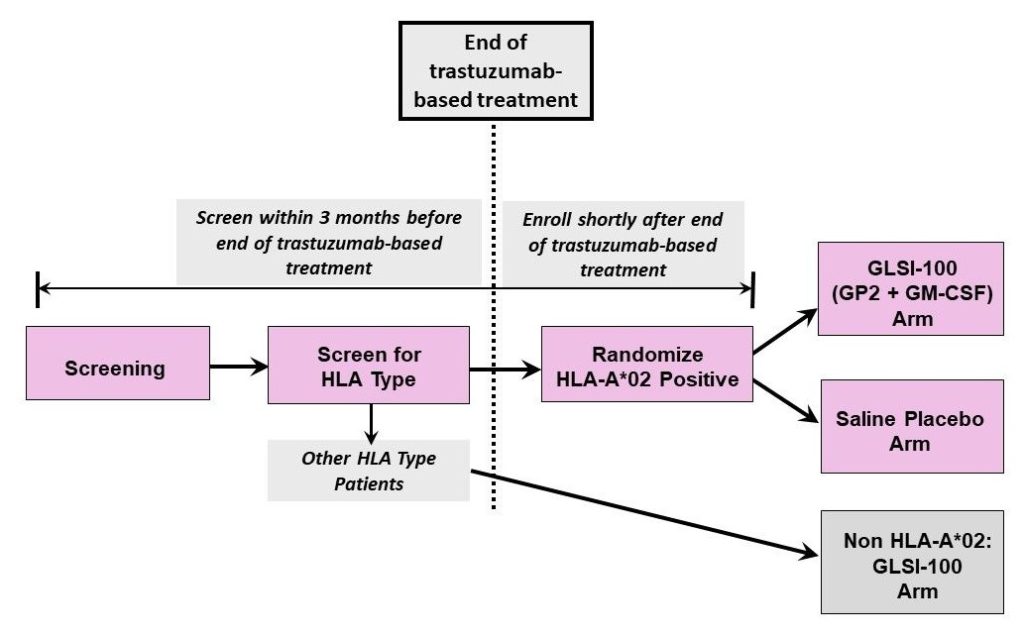
Flamingo-01 Trial Design
Patient Population
The Flamingo-01 Phase III trial is a prospective, randomized, double-blinded, multi-center study. The patient population is defined by major screening criteria and stratified in order to balance the patient population between the treated and placebo arms of the trial.
Major Screening Criteria:
- HER2/neu Positive Breast Cancer
- HLA-A*02 Positive
- Residual disease post neo-adjuvant therapy and surgery
- pCR if Stage III at presentation
Stratified By:
- Residual disease/pCR
- Hormone receptor status
- Geographic region
As currently designed, approximately 500 HLA-A*02 patients will be randomized to receive GLSI-100 or placebo in the first two pivotal arms of the trial, with a planned interim analysis. The planned interim analysis is conservatively designed for a 70% reduction in breast cancer recurrence, based on the prior Phase IIb results that showed an 80% or greater reduction in recurrence rate, in comparison to the ~50% recurrence reduction achieved by existing HER2-targeted therapies like Herceptin and Kadcyla. The primary endpoints of the pivotal arms will be blinded, but various other data — such as immune response and safety — may be open-label. In addition, up to 250 non HLA-A*02 patients will be enrolled in the third open-label arm, where all patients will receive GLSI-100 and where all endpoints will be open-label.
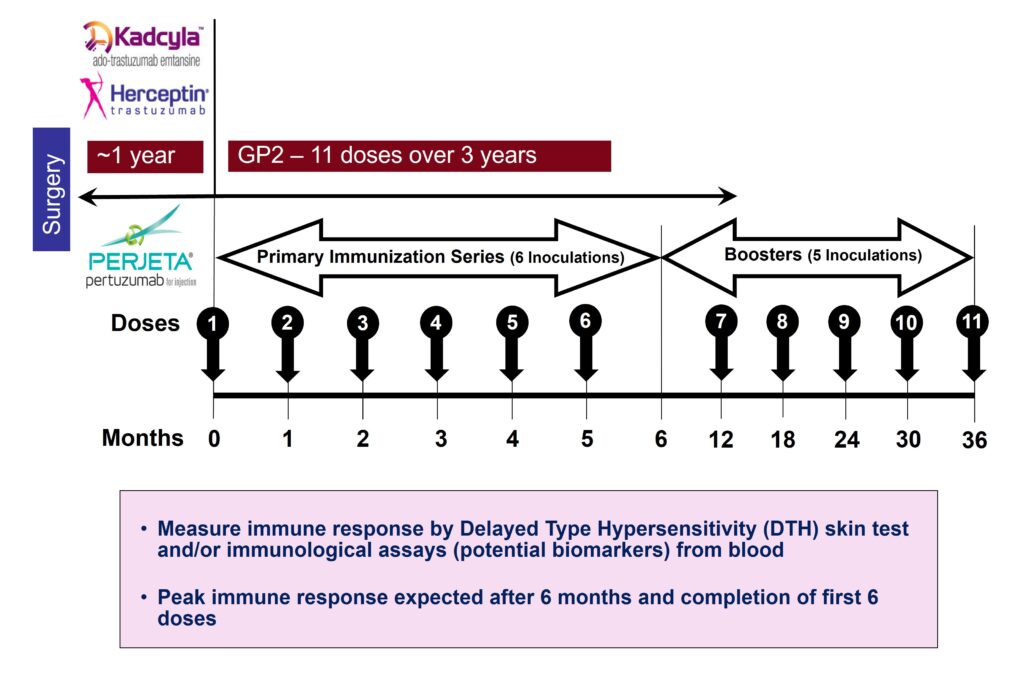
Treatment Regimen
After 1 year of trastuzumab-based therapy or an approved biosimilar, the patient duration will be 4 years, with a total of 11 injections over 3 years of treatment.
- First 6 Months of Treatment: The Primary Immunization Series (PIS), consisting of 6 intradermal injections of GLSI-100 or saline placebo, will be administered. The objective of the PIS is to provide protection against breast cancer recurrence after immunity has peaked at approximately 6 months. The objective of the PIS is to provide protection against breast cancer recurrence after immunity has peaked at approximately 6 months.
- Next 2.5 Years of Treatment: Five Boosters will be administered. The objective of the Boosters is to maintain immunity and thereby protection for longer periods of time.
- 1 Year of Follow-Up with No Treatment: Five Boosters will be administered. Immune response analysis is planned at baseline and various timepoints.
Phase III Clinical Trial Design — SABCS 2024
Poster P5-07-22: GP2 Phase III Clinical Trial Design for Preventing Breast Cancer Recurrence (view PDF here)